What We Accomplished in 2021-2022
Network Recruitment
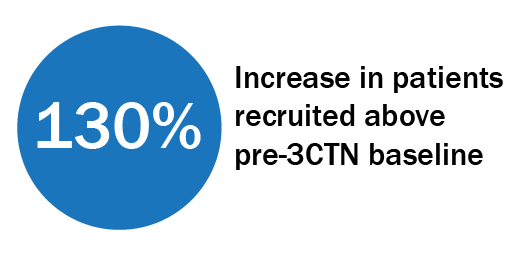

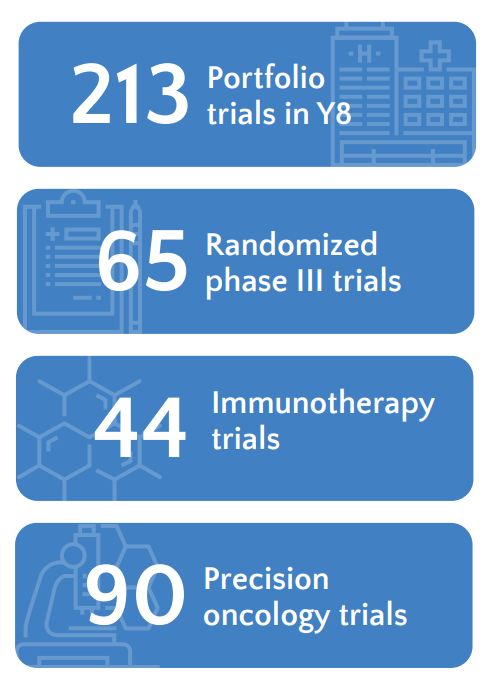
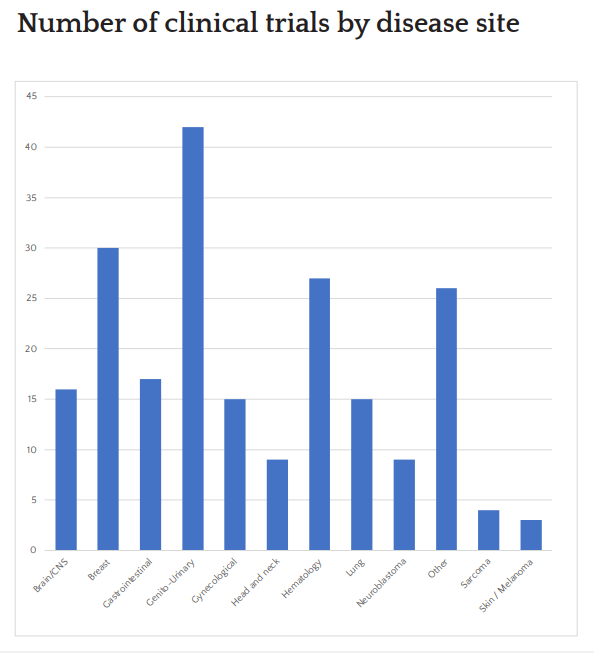
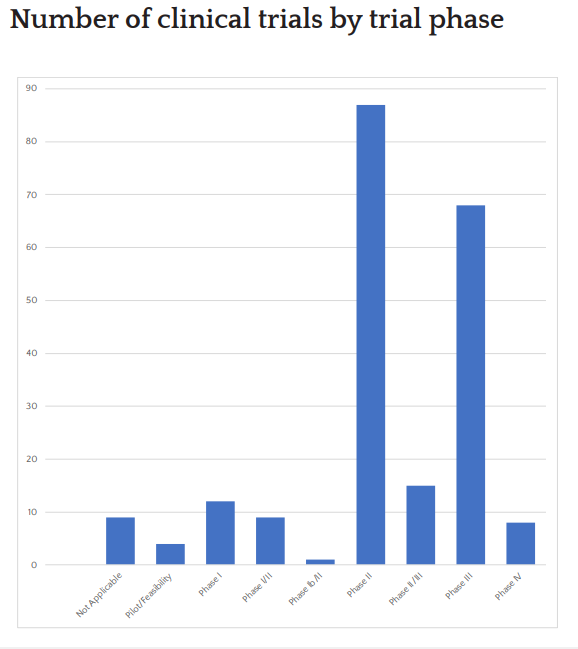
Trial Portfolio
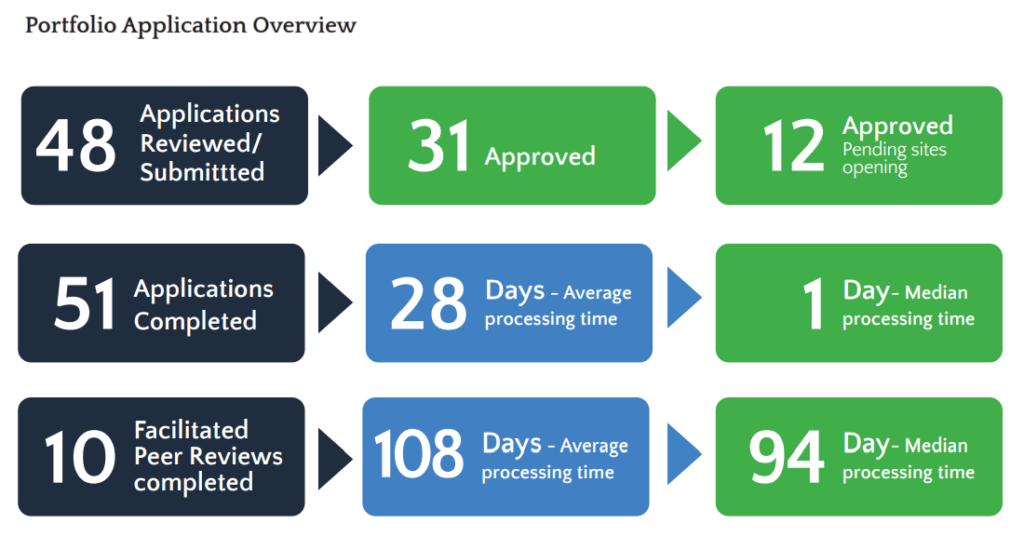
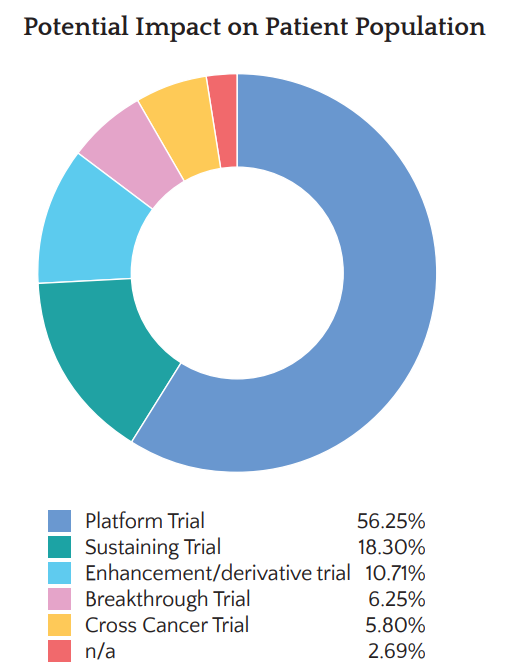
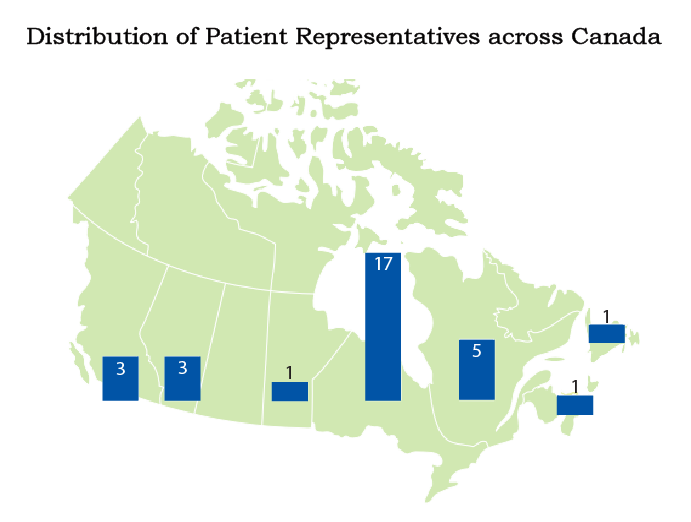
Patient and Public Involvement
Performance by Province
Alberta
Accrual to target 91% | 3 Patient partners
- Implemented a new Clinical Trials Management System (CTMS) that will result in improved trial efficiency.
- Engaged Patient Advisors in the implementation of a new electronic medical record (EMR) system. Patients can now be contacted via MyChart for potentially eligible trials.
- Training courses to address biosafety in clinical trials for staff members is under development.
British Columbia
Accrual to target 84% | 3 Patient partners
- Linked the new clinical trials website to the CTMS to provide an updated list of open trials for patients.
- Implemented a new Phase 1 room to allow patients on Phase 1 clinical trials to rest post-treatment.
Manitoba
Accrual to target 34% | 1 Patient partner
- Launched a new business unit model to allow greater physician engagement and more access to trials for patients.
- Held daily virtual events during International Clinical Trials Week and engaged Patient Representative to participate in events.
Newfoundland
Accrual to target 38% | 1 Patient partner
- Launched and implemented Clinical Trials Management System – EDGE.
- Listed all open trials on Eastern Health website for patients and stakeholders.
Nova Scotia
1 Patient partner
- Developed a Clinical Trials Working Group (CTWG) to address trial start-up, feasibility and patient population for recruitment.
- Added a Patient Representative to the CTWG to provide feedback on potential upcoming trials
Ontario
Accrual to target 151% | 17 Patient partners
- Developed clinical trial resources to support patients, families and friends.
- Implemented initiatives to improve trial selection and trial activation processes.
- Developed quality improvement initiatives to standardize processes and empower individuals to solve problems and integrate quality improvement into day-to-day work.
- Collaborated with other Network sites on best practices for audits.
Quebec
Accrual to target 193% | 5 Patient partners
- Developed and launched Clinical Trials Navigator pilot project, a first of its kind in the province.
- Redesigned and relaunched a new version of OncoQuébec, an online resource for sourcing available cancer clinical trials within the province of Quebec, based on user feedback.
Pediatrics
14 Patient partners
- C17 launched and promoted U-Link, a resource to improve access to clinical trials among the pediatric cancer sites.
- Three High Priority Trials (HPT) were identified and prioritized for trial activation. 94 per cent of participating sites activated HPTs within 90 days.

